Italy limits use of AstraZeneca vaccines to over-60s
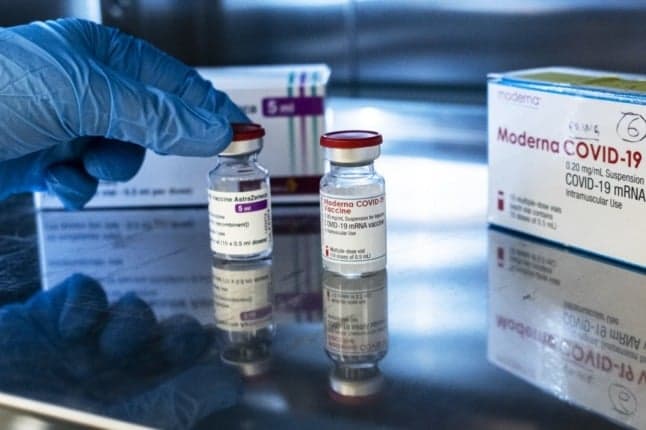
Italy's health ministry said on Saturday it would restrict the AstraZeneca vaccine to the over-60s, with younger people who have already received one dose to complete the cycle with Pfizer or Moderna.
The change follows an improvement in coronavirus infection rates in Italy, which has been devastated by the pandemic but will next week lift restrictions in much of the country following a sharp decline in cases.
The EU's medicine agency in April drew a link between rare blood clots and AstraZeneca jabs but stressed that the benefits of the vaccines outweighed the risks.
"The changed epidemiological situation has led to a reassessment of the risk-benefit ratio for age groups less at risk of severe forms of Covid-19," stated the Italian government's Technical and Scientific Committee (CTS), the panel which advises the government on health measures, on Friday.
A health ministry statement published on Saturday following the CTS review said that AstraZeneca's vaccine should now be given "only to people aged 60 or over".
Out of a principle of "maximum caution", for the under-60s who have already received the first dose of AstraZeneca, "the cycle should be completed with a second dose of mRNA vaccine" such as Pfizer-BioNTech or Moderna, administered eight to 12 weeks later.
READ ALSO: Is Italy really going to offer vaccines to tourists this summer?
The decision to give AstraZeneca only to over-60s "will have some effect on the vaccination campaign, but I'm sure that in July and August we will be able to mitigate this impact," Emergency Commissioner Francesco Figliuolo said at the press conference announcing the panel's findings on Friday.
Italy in March blocked use of the AstraZeneca jab over health fears, but after the EU's medicines agency gave the green light, approved it for everyone over 18.
It recommended preferential use for people over the age of 60, but AstraZeneca has been used for younger people, including in popular "open days".
Italy has so far administered 41 million vaccines and almost 14 million people out a population of around 60 million are fully vaccinated.
Photo: Marco BERTORELLO/AFP
AstraZeneca remains approved for adults of all ages in Italy, but the new advice strengthens a previous Health Ministry recommendation for “preferential use of AstraZeneca on people over the age of 60” due to a small number of cases worldwide of unusual blood clots in younger people who had recently received the vaccine.
Despite the previous advice to use the vaccine on over-60s, a number of Italian regions have been offering the Covid-19 vaccine to younger adults as part of vaccination "open days": special vaccination drives, often at evenings or weekends, that allow the youngest age groups to get a shot earlier than they otherwise might.
In most cases the doses available at these drives are AstraZeneca, also known as Vaxzevria.
Several regions have found themselves with unused doses of the Oxford University vaccine, as people opt to get vaccinated with alternatives that they perceive as safer or that have a shorter interval between doses (or in the case of Johnson & Johnson, require just one shot).
EXPLAINED: Why has Italy recommended the AstraZeneca vaccine for over-60s only?
The CTS gave the green light to AstraZeneca “open days” in May, when it said it did not object to regions offering the vaccine to over-18s on a voluntary basis.
To date, most of the rare cases of blood clots observed after vaccination with AstraZeneca or Johnson & Johnson have been reported in younger adults, prompting some EU countries to restrict their use to older age groups.
The European Medicines Agency (EMA) says it is not yet clear what the risk factors are, and that the benefits of the AstraZeneca vaccine continue to outweigh the very low chance of side effects.
There was also alarm after two young women, one aged 18 and another 34, were admitted to intensive care in Genoa this week with blood clots, around a fortnight after receiving a first dose of AstraZeneca from the same batch.
The younger woman died on June 10th, local authorities announced.
The regional health authority has withdrawn the batch in question throughout Liguria as a precautionary measure, it said on Thursday.
AstraZeneca vaccination drives remain underway or scheduled in several parts of Italy, including Lazio, the region around Rome, which is currently running an AstraZeneca "open week" for over-18s until June 13th.
READ ALSO:
- ‘Be tenacious as hell’: How people in Italy have managed to get vaccinated without a health card
- How can you book a Covid vaccination appointment in Italy?
- The essential Italian vocab for getting tested or vaccinated for Covid-19
Around a third of all doses of AstraZeneca and Johnson & Johnson administered in Italy in the past three weeks – more than 470,000 – went to under-50s, according to GIMBE.
Most of the side effects observed so far occurred within two weeks of the first dose. The second dose is thought to carry an even lower risk, though more data is needed to know for sure.
Data from millions of people vaccinated with AstraZeneca across Europe and the UK indicates that serious side effects after either dose remain extremely rare.
In a monitoring report released this week, AIFA said that in Italy the incidence of blood clots after vaccination was around one per 100,000 injections of AstraZeneca, mainly in people under 60. It has not received reports of any clots developing after the second dose, the agency said.
“All vaccines are safe,” Health Minister Roberto Speranza told parliament on Thursday, while confirming that the CTS was reviewing its advice on the use of AstraZeneca in young people.
What symptoms should people watch out for?
The EMA advises seeking medical help immediately if you notice any of these symptoms after getting vaccinated:
- shortness of breath
- chest pain
- swelling in your leg
- persistent abdominal pain
- neurological symptoms, including severe and persistent headaches or blurred vision
- tiny blood spots under the skin beyond the site of injection
Early medical treatment can prevent complications and help lead to recovery, the agency says.
Comments (1)
See Also
The change follows an improvement in coronavirus infection rates in Italy, which has been devastated by the pandemic but will next week lift restrictions in much of the country following a sharp decline in cases.
The EU's medicine agency in April drew a link between rare blood clots and AstraZeneca jabs but stressed that the benefits of the vaccines outweighed the risks.
"The changed epidemiological situation has led to a reassessment of the risk-benefit ratio for age groups less at risk of severe forms of Covid-19," stated the Italian government's Technical and Scientific Committee (CTS), the panel which advises the government on health measures, on Friday.
A health ministry statement published on Saturday following the CTS review said that AstraZeneca's vaccine should now be given "only to people aged 60 or over".
Out of a principle of "maximum caution", for the under-60s who have already received the first dose of AstraZeneca, "the cycle should be completed with a second dose of mRNA vaccine" such as Pfizer-BioNTech or Moderna, administered eight to 12 weeks later.
READ ALSO: Is Italy really going to offer vaccines to tourists this summer?
The decision to give AstraZeneca only to over-60s "will have some effect on the vaccination campaign, but I'm sure that in July and August we will be able to mitigate this impact," Emergency Commissioner Francesco Figliuolo said at the press conference announcing the panel's findings on Friday.
Italy in March blocked use of the AstraZeneca jab over health fears, but after the EU's medicines agency gave the green light, approved it for everyone over 18.
It recommended preferential use for people over the age of 60, but AstraZeneca has been used for younger people, including in popular "open days".
Italy has so far administered 41 million vaccines and almost 14 million people out a population of around 60 million are fully vaccinated.

AstraZeneca remains approved for adults of all ages in Italy, but the new advice strengthens a previous Health Ministry recommendation for “preferential use of AstraZeneca on people over the age of 60” due to a small number of cases worldwide of unusual blood clots in younger people who had recently received the vaccine.
Despite the previous advice to use the vaccine on over-60s, a number of Italian regions have been offering the Covid-19 vaccine to younger adults as part of vaccination "open days": special vaccination drives, often at evenings or weekends, that allow the youngest age groups to get a shot earlier than they otherwise might.
In most cases the doses available at these drives are AstraZeneca, also known as Vaxzevria.
Several regions have found themselves with unused doses of the Oxford University vaccine, as people opt to get vaccinated with alternatives that they perceive as safer or that have a shorter interval between doses (or in the case of Johnson & Johnson, require just one shot).
EXPLAINED: Why has Italy recommended the AstraZeneca vaccine for over-60s only?
The CTS gave the green light to AstraZeneca “open days” in May, when it said it did not object to regions offering the vaccine to over-18s on a voluntary basis.
To date, most of the rare cases of blood clots observed after vaccination with AstraZeneca or Johnson & Johnson have been reported in younger adults, prompting some EU countries to restrict their use to older age groups.
The European Medicines Agency (EMA) says it is not yet clear what the risk factors are, and that the benefits of the AstraZeneca vaccine continue to outweigh the very low chance of side effects.
There was also alarm after two young women, one aged 18 and another 34, were admitted to intensive care in Genoa this week with blood clots, around a fortnight after receiving a first dose of AstraZeneca from the same batch.
The younger woman died on June 10th, local authorities announced.
The regional health authority has withdrawn the batch in question throughout Liguria as a precautionary measure, it said on Thursday.
AstraZeneca vaccination drives remain underway or scheduled in several parts of Italy, including Lazio, the region around Rome, which is currently running an AstraZeneca "open week" for over-18s until June 13th.
READ ALSO:
- ‘Be tenacious as hell’: How people in Italy have managed to get vaccinated without a health card
- How can you book a Covid vaccination appointment in Italy?
- The essential Italian vocab for getting tested or vaccinated for Covid-19
Around a third of all doses of AstraZeneca and Johnson & Johnson administered in Italy in the past three weeks – more than 470,000 – went to under-50s, according to GIMBE.
Most of the side effects observed so far occurred within two weeks of the first dose. The second dose is thought to carry an even lower risk, though more data is needed to know for sure.
Data from millions of people vaccinated with AstraZeneca across Europe and the UK indicates that serious side effects after either dose remain extremely rare.
In a monitoring report released this week, AIFA said that in Italy the incidence of blood clots after vaccination was around one per 100,000 injections of AstraZeneca, mainly in people under 60. It has not received reports of any clots developing after the second dose, the agency said.
“All vaccines are safe,” Health Minister Roberto Speranza told parliament on Thursday, while confirming that the CTS was reviewing its advice on the use of AstraZeneca in young people.
What symptoms should people watch out for?
The EMA advises seeking medical help immediately if you notice any of these symptoms after getting vaccinated:
- shortness of breath
- chest pain
- swelling in your leg
- persistent abdominal pain
- neurological symptoms, including severe and persistent headaches or blurred vision
- tiny blood spots under the skin beyond the site of injection
Early medical treatment can prevent complications and help lead to recovery, the agency says.
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.